Formation of NOx Compounds in CH4 Combustion
This problem considers the design of the combustion component of an industrial boiler which
burns methane in air. The goal is to choose the feed composition (air:fuel ratio) in such a
way that NOx compound formation is inhibited, while maintaining a sufficiently
high flame temperature. With EQS4WIN, you can perform similar calculations using any other fuel
of interest.
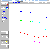
This problem explores a different aspect of the example combustion problem that appears on the list of Example Applications. discussed elsewhere. Briefly, that example considers the combustion of CH4 in air, as a function of air:fuel ratio and the temperature. Adiabatic flame temperatures of the product gas are determined, in addition to the full product gas species distribution. The calculation considers a total of 44 species in the product gas, including various environmentally polluting NOx compounds. This enables one to quickly explore the effect of the air:fuel ratio on both the flame temperature and the product composition with respect to these compounds. You will find that the equilibrium amounts of these products can be significantly decreased by altering the fuel composition away from the stoichiometric value used to achieve complete combustion, while at the same time not significantly altering the flame temperature.
The example problem included in all versions of EQS4WIN explores the results of a more accurate calculation for such a problem in three ways. First, complete combustion is not assumed, and the full equilibrium composition and enthalpy change is calculated as a function of temperature. Second, the air:fuel ratio is varied to examine the effects of this quantity on the final results. Finally, you can investigate the effects of different temperatures of each species in the inlet feed-stream, simply by clicking on the Heat Balance button and editing the data. In all, a total of 44 species are included in the calculation. An important consequence of this is the availability of the complete composition of the product gas.
Click on image to enlarge (15K)